

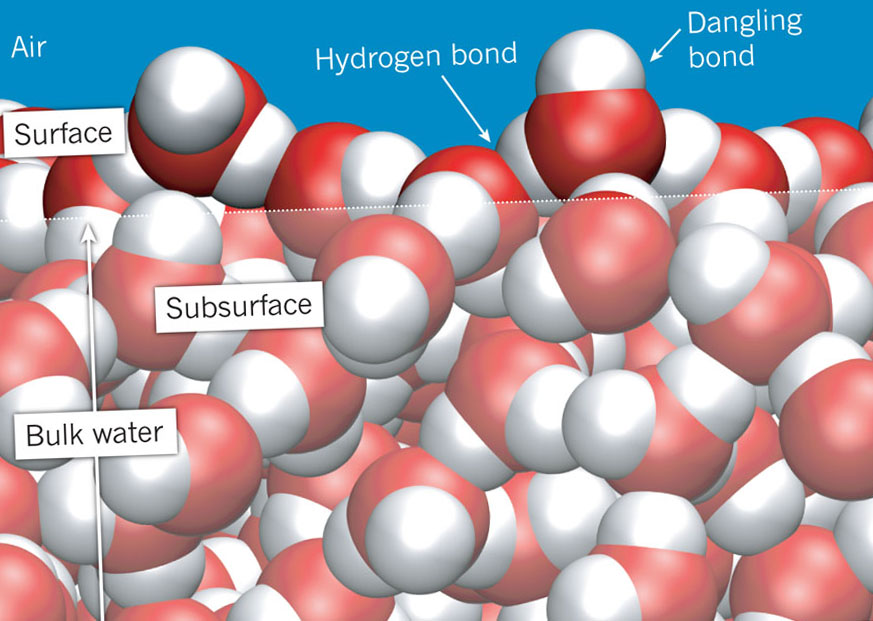
While in each case the classical mean-field theories can explain many macroscopic and mesoscopic observations, it soon becomes apparent that such theories fail to explain phenomena for which molecular properties are relevant, such as interfacial chemical conversion. In this Review, by considering water in contact with metals, oxides and biomembranes, we show the essential similarity of these disparate systems. This diversity in substrates has led to different communities considering each of these types of aqueous interface. While water, solutes and charge are present in each of these systems, the substrate can range from living tissues to metals. Even so, many open questions remain regarding the molecular picture of the interfacial organization and preferential alignment of water molecules, as well as the structure of water molecules and ion distributions at different charged interfaces. This "bipolar" nature of water molecules gives water its cohesive nature, and thus, its stickiness and clumpability (maybe "dropability" is a better term?).The ubiquity of aqueous solutions in contact with charged surfaces and the realization that the molecular-level details of water–surface interactions often determine interfacial functions and properties relevant in many natural processes have led to intensive research. Thus when the positive side on one water molecule comes near the negative side of another water molecule, they attract each other and form a bond. In a water molecule, the two hydrogen atoms align themselves along one side of the oxygen atom, with the result being that the oxygen side has a partial negative charge and the side with the hydrogen atoms has a partial positive charge. Molecular layout of liquid water molecules Opposite magnetic poles attract one another much like positively charged atoms attract negatively charged atoms in water molecules. If you've played with bar magnets you will know that the north pole of one magnet will repel the north pole of another magnet, but it will attract the south pole of another magnet. More precisely, the positive and negative charges of the hydrogen and oxygen atoms that make up water molecules makes them attracted to each other. Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and electricity are involved at a more detailed level to make this possible. Water is highly cohesive-it is the highest of the non-metallic liquids. Sources/Usage: Some content may have restrictions. Although you may have heard of a "skin" where water meets the air, this is not really an accurate description, as there is nothing other than water in the drop. On Earth, the effect of gravity flattens this ideal sphere into the drop shape we see.
For water, this state happens when a water molecule is surrounded on all sides by other water molecules, which creates a sphere or ball (perfectly round if it was in outer space). The natural form of a water drop occurs during the "lowest energy state", the state where the atoms in the molecule are using the least amount of energy. It turns out that this surface tension is the result of the tendency of water molecules to attract one another. If you just look at the picture of the water drop sitting of the leaf, you might think the water drop has a "skin" holding it into a sort of flattened sphere (although there is nothing flat about a water drop in outer space). Adhesion and cohesion are winning the battle so far, as the drops are sticking to the pine needles. Gravity is working against both adhesion and cohesion, trying to pull the water drop downward. Also noticeable in this picture is the effect that gravity has on the water drops. In the picture of pine needles above, the water droplets are stuck to the end of the pine needles-an example of the property of adhesion.

Schmidt, National Park ServiceĪ water drop is composed of water molecules that like to stick together-an example of the property of cohesion.


 0 kommentar(er)
0 kommentar(er)
